 |
Fig. 1 (ab) TEM images of partially crystallized Ni3(BO3)2 coated Ni3B nanoparticles (pc-Ni-Bi@NB); (c) Comparison of OER activity of catalysts reported in pc-Ni-Bi@NB and literature (de) Ni3B-coated Ni3B with different degrees of crystallinity, an oxygen evolution TOF curve and a TOF value at an overpotential of 300mV (1: amorphous; 2-5: crystallinity increases sequentially).
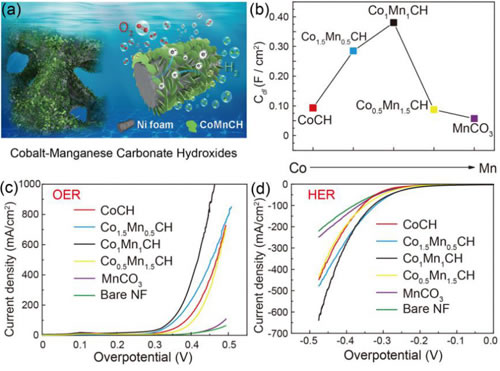
Fig. 2 (a) Schematic diagram of manganese-doped basic cobalt carbonate (CoxMnyCH) nanosheet array electrolyzed water catalyst; (b) Diagram of electrochemical activity area of ​​CoxMnyCH catalyst versus Co/Mn ratio; (c) Analysis of CoxMnyCH catalyst Oxygen polarization curve; (d) Hydrogen evolution polarization curve of CoxMnyCH catalyst.
Hydrogen energy is an ideal energy carrier. Developing large-scale, cheap, clean and efficient hydrogen production technology is the key to the effective use of hydrogen energy. Electrolyzed water is one of the green hydrogen production methods with promising applications because of its environmental friendliness, high product purity, and non-carbon emissions. The most important bottleneck for limiting the large-scale application of electrolysis water to hydrogen production is how to significantly reduce its electric energy consumption, thereby significantly reducing the cost of hydrogen production. The key point is how to effectively reduce the overpotential of oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) on the electrode, and realize hydrogen production at high current under low cell pressure. Therefore, the development of cheap, easy-to-prepare, high-efficiency electrolyzed water catalysts has attracted attention.
Under the support of the National Natural Science Foundation of China, the Ministry of Science and Technology, and the China National Academy of Sciences' Strategic Leading Science and Technology Project, Research Scientist Hu Jinsong, Research Fellow at the Key Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences, has conducted research on the clean acquisition and application of hydrogen energy. A series of studies was carried out (ACS Nano 2016, 10, 851; Nano Energy 2016, 28, 319; Adv. Sci. 2017, 4, 1700084; J. Am. Chem. Soc. 2016, 138, 3570). Recently, in response to the problem of slower specific kinetics and higher overpotential for the anodic oxygen evolution reaction in electrolyzed water, they found that the oxygen evolution catalyst is environmentally-friendly and has a microscopic morphology and electronic structure. It can greatly increase its electrocatalytic oxygen evolution activity and stability, providing another way to expand the choice of electrolytic water electrode materials. They found that the electrocatalytic oxygen evolution performance of nickel(II) borate (Ni3(BO3)2) nanocatalyst was closely related to its crystallinity, and through the regulation of its crystallinity, a novel nickel(II) borate analysis with excellent OER performance was obtained for the first time. Oxygen electrocatalyst (Figure 1). The results of this fine tuning of the performance of electrocatalysts by regulating the degree of crystallinity of electrocatalysts provide new ideas for the development of new low-cost, high-efficiency electrocatalysts. The results of relevant studies were recently published on Angew. Chem. Int. Ed. 2017, 56, 6572 and reported by the Chinese Journal of Physical Chemistry as highlight.
In addition, the basic carbonate preparation method is simple, economical and environmentally friendly, but its performance of electrocatalytic water decomposition is not good. Researchers recently discovered that by introducing manganese into basic cobalt carbonate, the dual control of its micro-morphology and electronic structure can be achieved, and its electro-catalytic water decomposition performance can be greatly improved, making basic carbonates that have no prospect of application. The material becomes a new dual-function fully hydrolyzed electrocatalyst that can work with high currents comparable to recently reported high-performance sulfides, phosphides, etc. (Fig. 2). This study not only broadened the range of options for low-cost, high-efficiency electrolyzed water catalysts, but also helped to understand the structure-activity relationship of the catalysts and provided new ideas for the development of new, environmentally friendly, practically-efficient, low-cost electrocatalysts. The results of the relevant studies are published on J. Am. Chem. Soc. 2017, 139, 8320.
Antimony Iii Oxide,Diantimony Trioxide,Pure Diantimony Trioxide,Antimony Trioxide 99.8% Min
HUNAN ZHONGNAN ANTIMONY&TUNGSTEN TRADING CO.,LTD , https://www.znat.com.cn